|
|
 |
 |
|
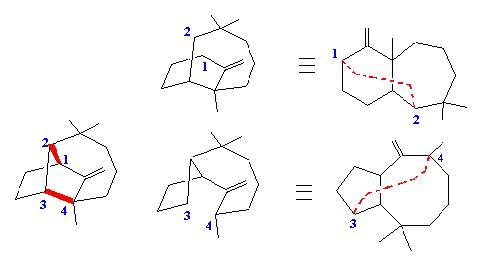
SAS (Simulated Analytical Synthesis). The retrosynthetic approach described for SOS tries to find the solutions step by step. It is interesting and allows one to discover original syntheses, but when a chemist looks at a structure, he doesn’t consider all possibilities. He selects rapidly the bonds to be made (or broken in the retrosynthetic sense). In his synthesis of Longifolene, Corey (JACS, 1964, 86, 478) proposed the analytical approach which tries to find the strategic bonds. These bonds are those which generate simple precursors when they are deleted. The following example illustrates this approach in the case of Longifolene. Two strategic bonds are displayed in red, if we delete them we obtain potential precursors, and the task of the chemist is to find a synthesis involving the formation of these bonds as the key-step.
|
 |
 |
|
We wrote the program SAS which aim was the delete one or two bonds of the skeleton of the target.
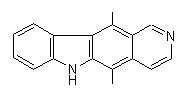
Ellepticine was selected as the target molecule :
|
 |
 |
|
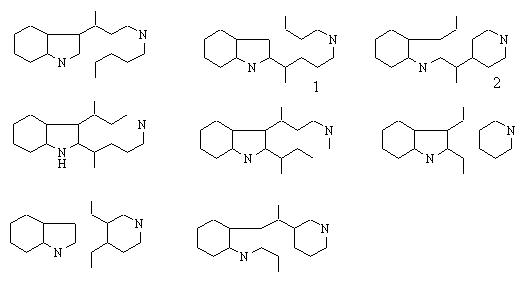
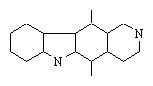
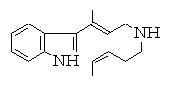
Deleting two bonds leads to 253 solutions ! We show here some which suggest Diels-Alder reaction :
|
 |
 |
|
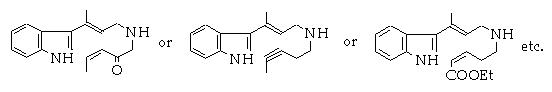
The interest of this approach is that it suggests the key step of the synthesis,
|
 |
 |
|
Then the chemist has to improve this idea, for example :
|
 |
 |
|
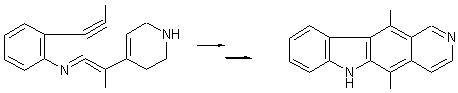
A synthesis involving scheme 2 has been experimented by Prof. Ghosez (Tet. Lett., 1985, 26(13), 1647) :
|
 |
 |
|
As one can see this simple approach can suggest interesting strategies. The main drawback is that it can’t generate solutions involving fragmentations or rearrangements. It’s why we developed REKEST then HOLOWin.
|
 |
 |
|
Papers about SAS :
9 - Ordinateur et synthèse organique. Approche analytique. Exemple de l'azaadamantane.
R. Barone, A. Boch, M. Chanon and J. Metzger
Computers and Chemistry, 1979, 3, 83-87.
14 - Synthesis of ellipticine. Review and computer suggestions.
R. Barone and M. Chanon
Heterocycles, 1981, 16, 1357-1365.
|
|