|
|
 |
 |
|
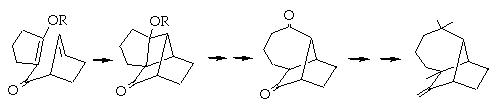
The idea of this program was to improve SAS which was unable to generate rearrangements or fragmentation. For example if we consider the synthesis of Longifolene by Oppolzer (J. Am. Chem. Soc., 1978, 100, 2583) in which the key step involves an intramolecular de Mayo reaction ( (2+2 ) then retro aldol) :
|
 |
 |
|
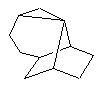
With SAS the following precursor could eventually suggest this idea :
|
|
|
 |
 |
|
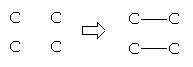
So we decided to adapt SOS where we coded “pseudo reactions” which add and / or delete bonds in the skeleton of the target, such as :
|
 |
 |
|
Reaction which adds and deletes two bonds :
|
 |
 |
|
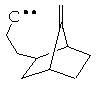
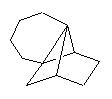
For Longifolene framework, adding one bond generates several solutions, among them :
|
|
|
 |
 |
|
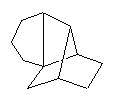
It suggests a similar strategy, which is new :
|
|
|
|
|
 |
 |
|
Could suggest the synthesis of Longifolene by Oppolzer.
|
 |
 |
|
The main drawback is that it generates a great number of solutions. The search for the key step of a synthesis was improved with CONAN and HOLOWin programs.
|
 |
 |
|
Paper :
26 - REKEST : a computer program to REsearch for the KEy STep of a synthesis
R. Barone and M. Chanon
Chimia, 1986, 12, 436-439.
|
|