|
|
||||||||||||
|
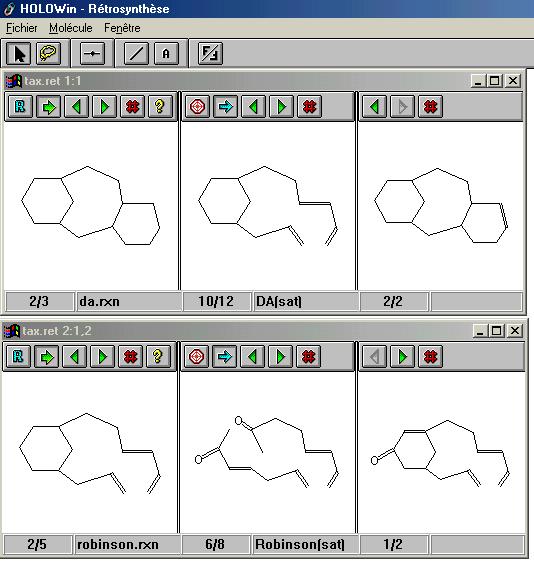
HOLOWin (HOLOsynthon and Windows) : the aim of this program is to search for the key steps of a synthesis. |
|
|
||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
 |
|
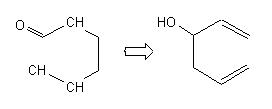
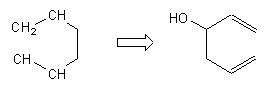
and for each target the oxy-Cope reaction is applied, which leads, among other solutions, to the interesting ones : |
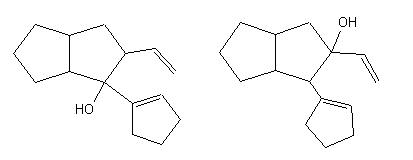 |
|
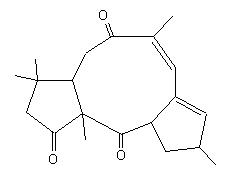
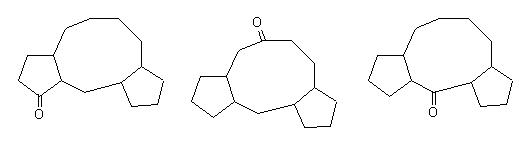
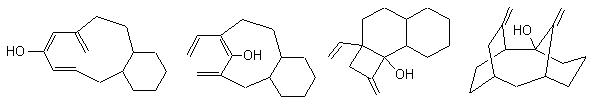
With this appraoch the chemist can see very quickly if a given reacion has some chances to be used to built the skeleton of the target. The problem is not solved but the main direction is given. Below are some examples for the following targets of Taxol© : |
 |
|||||
|
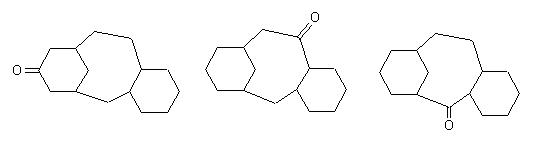
Some possible precursors : |
|||||
 |
|||||
|
Solution 1 was published in 1989 (Tetrahedron, 1989, 45, 1985 pub. n°31) and was tested independently next year by another searchors (Zücker, Lupia, Syn. Let. 1990, 729) |
|
|
||||||||||||||||||||||||||||||||
|
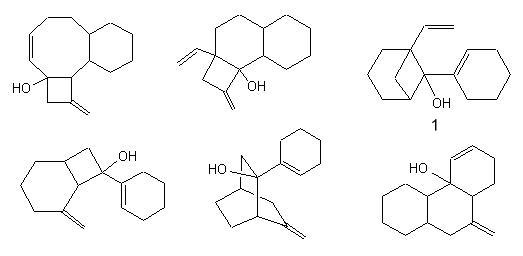
And the solutions are : |
 |
|
This approach is very interesting, the only drawback is that in some cases we are obliged to submit several structures for one target. So we decided to generalize it by coding targets and reactions at a skeletal level. All functions are removed. |
|
|
||||||||||||||||||||||||||||
|
By this way all the possibilities are found, 72 for the Taxol, but it is difficult to visualize where the functions are located in the target. So we improved this approach by coding the reaction in two steps : backward and forward (this one being fully functionalized). Then in the program we added an option which allows to perform the forward reaction on the precursors. |
|
Example. |
|
|
||||||||||||||||
|
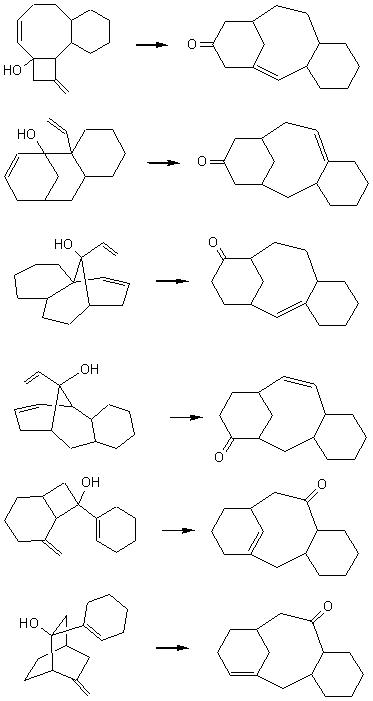
Some solutions for the Taxol skeleton : |
|
|
 |
|
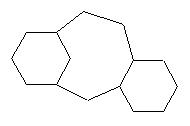
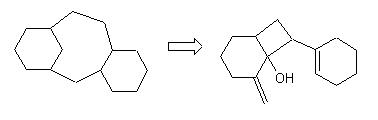
For the crinipellin framework, from radical cascade reaction, this appraoch led to several solutions, and among them these ones which were new : |
 |
||||||||||||||||||
|
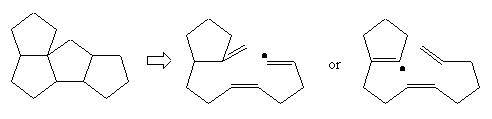
They suggest a new radicalar cyclic pathway which was tested successfully by Prof. Curran from our suggestion (Aust. J. Chem, 1995, 48, 261-267) : |
||||||||||||||||||
 |
||||||||||||||||||
|
This approach was first developed as an option in the Marseil/SOS program (running on a Macintosh), then we improved it in the HOLOWin software for the Windows interface. In this version we added an important option : when a precursor is displayed on the screen it is possible to analyze it directly in a new window, then come back to the parent target. (In the previous version the precursors were saved on disk, then when all precursors have been displayed, the user could select one of them as a new target and analyzes it). |
||||||||||||||||||
 |
||||||||||||||||||
|
In the forward direction the strategy should be : |
||||||||||||||||||
 |
||||||||||||||||||
|
Strategies proposed for Taxol from [2+2+2], Diels Alder and Robinson reactions : |
||||||||||||||||||
 |
||||||||||||||||||
|
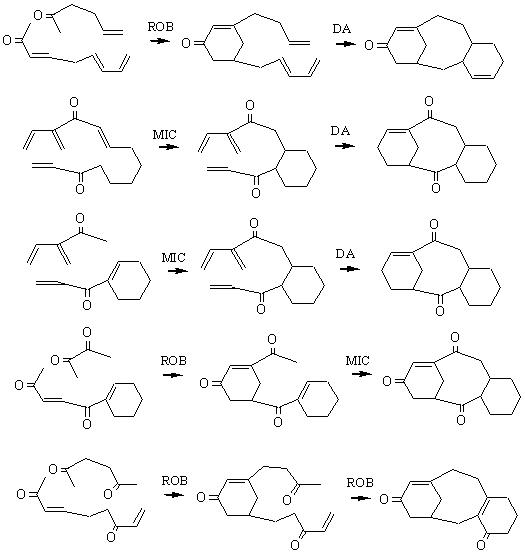
Some other strategies proposed by Holowin for the Taxol framework from three reactions (Robinson, Diels-Alder, Michael) (they are shown in the forward direction) : |
||||||||||||||||||
 |
||||||||||||||||||
|
Related papers : 31 - How to search original key steps in a synthesis of complex structures by using a microcomputer as a pocket-like calculator. 35 - Microcomputer Assisted RetroSynthesis. 38 - New computer-based approach for seeking a key step in the synthesis of complex structures. Application to Taxane and Crinipellin diterpenoid frameworks. 39 - La conception de stratégies de synthèse par ordinateur : le logiciel STRAKS 41 - Search of the key step of a synthesis with STRAKS program. Examples with the taxane skeleton. 45 - Computer-Aided Organic Synthesis : The Holosynthon Concept and the HOLOWin software. 46 - HOLOWin : A Fast Way to Search for Tandem Reactions with Computer. Application to the Taxane Framework. 47 - Le Logiciel HOLOWin, une approche simple, rapide, originale pour rechercher des stratégies de synthèse par ordinateur. |
|
[Home] [Some dates] [Papers] [sos] [marseil] [conan] [holowin] [graal] [miscellaneous] |